Contract Development and Mnanufacturing Serivices
Globally accptable ”Knowledge" based on abundant experiences and
"Power" generated by highly specialized technologies
Shionogi Pharma can provide "Full-range Services" at "One Stop"
We can support our customers through our serivices including not only API process development, pharmaceutical development and commercial manufacturing of API and drug products but also analytical method development, equipment design based on pharmaceutical engineering techlologies, etc.
Our sercvices will satisify our customers' needs.
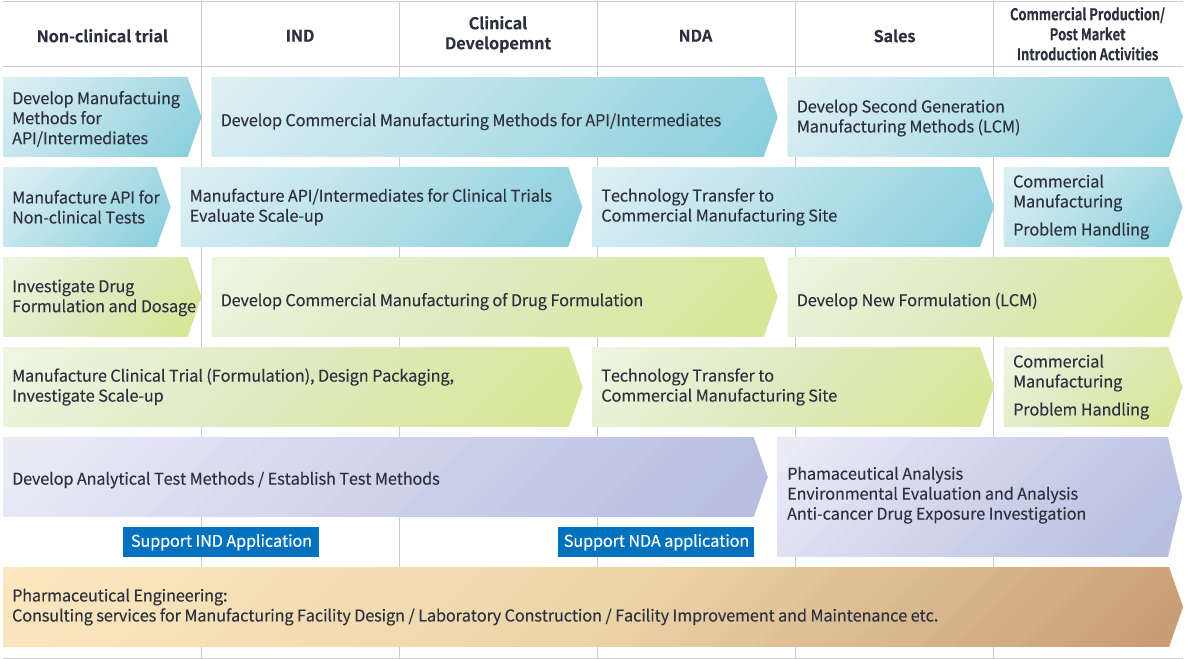
On-stop service from clinical trial to market introduction
-
One Stop Service
From non-clinical stage to commercial production and LCM (Life Cycle Management.) We are capable of providing a one-stop service for all operational aspects of CMC and general production related matters.
-
High Quality
We manufacture high quality clinical drugs and products at four manufacturing sites that have cleared audits from many country authorities.
-
Support
CMC R&D leveraging our experience as a drug development based pharmaceutical company.
We support creation of agency application documents and responses. -
Engineering
Based on our rich experience as a pharmaceutical manufacturer, we provide a full range of pharmaceutical engineering services covering basic design, validation and maintenance.
-
Full Range
We provide a full range of services including pharmaceutical analysis (establish analytical test methods, validation) that meet J-GMP, c-GMP and EU-GMP requirements, environmental evaluation & analysis, and additional services such as anti-cancer drug exposure investigation.
Full Range Services from Shionogi Pharma
A tightly knit linkage between the various processes is very critical to ensure the success of new drug development and to conduct manufacturing at a low cost.