API Manufacturing Site for Clinical Trial API to Commercial API
API Manufacturing Site for Clinical Trial API to Commercial API
Tokushima Plant has a complete line of equipment and has rich experience in pharmaceutical API manufacturing. Integrated manufacturing from clinical trial API to commercial API can be done at this plant. We can assure sense of security to our clients and enable smooth market introduction.

224-20 Hiraishi Ebisuno, Kawauchi-cho, Tokushima City, Tokushima 771-0132, Japan
TEL +81-88-665-2312

Japan
PMDA
USA
FDA
South Korea
MFDS
Russia
Minzsrav
API / Intermediates Manufacturing Facility Building
This is the manufacturing building of API for low molecular weight general pharmaceuticals. This production facility is capable of manufacturing investigational new drug to commercial production. This production facility is an ideal solution for clients contemplating comprehensive contract manufacturing from late development stage to commercial stage manufacturing.
The facility was built with Environmental/Health/Safety (EHS) in mind and by placing emphasis on the safety of workers, including segregation of dangerous materialusage areas and confinement measures against chemicals, the facility has been established to meets global standards. The facility has cleared many overseas agencies' audits.

A-2 Building

Reactor
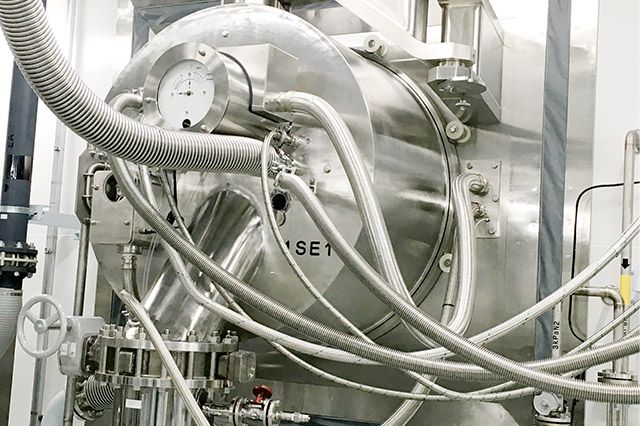
Centrifuge

Conical Dryer
This is the manufacturing facility of API for low molecular weight general pharmaceuticals. Based on our accumulated manufacturing technologies and rich contract manufacturing experience fostered over many years, we are manufacturing pharmaceutical API in compliance with GMP. We are capable of special manufacturing processes such as ozonation reaction, and with multiple units of counter jet mills, we can flexibly handle pulverizing in accordance with required manufacturing volume.

Counter Jet Mill 200AFG

Counter Jet Mill 100AFG
General Work Area Major Facilities
| Reactor | 500~6,000L(SUS,GL,MA22) |
|---|---|
| High Pressure Reactor | 6,000, 2,000L(GL) |
| Low Temperature Reactor | 1,500L(GL,-70)˚C |
| Separator | Centrifuge: 39inch(MA22),42inch(TL) |
| Pressurized Filtration Equipment | 3m2(SUS),2m2(TL),1m2(TL) |
| Dryer | Conical: 2,000 (GL), Through Gas Dryer (SUS) |
| Medium Tank | 3,000, 1,500, 500, 300L and others(SUS,GL) |
| Special Purpose Equipment | Ozone Generation Equipment:O3 40g/Nm3-96Nm3/h |
Product Area
| Reactors | 1,500~6,000L(GL,SUS) |
|---|---|
| Separator | Centrifuge:39inch(MA22),42inch(TL) |
| Dryer | Conical:1,000, 3,000L(GL) |
| Pulverizer | Power Mill, Hammer Mill (SUS), Counter Jet Mill 100AFG,200AFG(SUS) (nitrogen/air) |
High Pharmacological Activity API Manufacturing Facility Building
This is a multi-purpose plant capable of GMP manufacturing 1-10kg/lot of high pharmacological activity API. We have two manufacturing line that works independently and these systems are rated as Hazard Level 5 (OEL 0.1 to 1μg/m3.) This is an ideal facility for clients looking for a manufacturing facility capable of handling relatively small manufacturing volume of high pharmacological activity material.

Glove box for Preparation

Centrifuge

Filter Dryer

Counter Jet Mill (Isolator specification)
Dedicated Anti-cancer Drugs General Work Area Major Facilities
| Reactor / Distiller | 50~100L(GL) |
|---|---|
| Other Facilities | Rotary Evaporator: 30L Sterilized purified water production equipment |
Dedicated Anti-cancer Drugs Product Area Major Facilities
| Reactor | 50L(GL) |
|---|---|
| Other Facilities | Rotary Evaporator: 30L Isolator |
API General Work Area Major Facilities
| Preparation Equipment /High Pressure Reactor | 50L(GL) |
|---|---|
| High Pressure Reactor | 300L(GL) |
| Reactor/Distiller | 50~400L(SUS,GL,MA22) |
| Other facilities | Sterilized purified water production equipment |
API Product Area Major Facilities
| Crystallizer | 50, 300L(GL) |
|---|---|
| Pressurized Filtration Equipment | 0.2m2(TL) |
| Filtration Dryer | 0.2m2(MA22) |
| Separator | 25inch(MA22) |
| Pulverizer | Counter Jet Mill 100AFG (SUS) |
| Other Facilities | Isolator |
Spray Dryer Facility
As a way of improving solubility of poorly soluble drugs. the Spray Dryer handles the manufacturing of individual particle dispersion. This equipment can also handle organic solvents.

Dissolution Equipment

Coulter Dryer
Coulter Dryer CD-5 Type manufactured by Japan Chemical Engineering & Machinery
| Solvent Evaporation Volume | 40.0kg/H (Acetone 95%, Ethanol 5%) |
|---|---|
| Original Solution Processing Capacity | 44.0kg/h(max) |
| Spray Nozzle | High Pressure Revolving Spray Type |
| Hot Air intake Temperature | 120~140 ± 3˚C |
| Exhaust Air Outlet Temperature | Dependent on outcome (about 85˚C expected) |
| Material in Contact with Drug, Finish | SUS, Buffed #400 |
| Air Heating Method | Indirect Steam Heating |
| Fine Particle Collection Method | Bug Filter (Filter cloth: Heat resistant Polyester felt) |




