Integrated manufacturing site specializing in cancer pain treatment drugs, and cephem/carbapenem antibiotics
Integrated manufacturing site specializing in cancer pain treatment drugs, and cephem/carbapenem antibiotics
The capacity of Kanegasaki Plant is approximately 100 tons/year for antibiotics API, 17 million vials/year for vial formulation and 420 million tablets/year for tablet formulation, where manufacturing is conducted under adequate confinement and aseptic technology to guarantee high quality to meet safety requirements and to give a sense of security to clients. Our past performance of clearing many overseas and domestic audits can assure our clients to place trust on us and enable smooth market introduction of products.

7 Nishine Moriyama, Kanegasakai City, Iwasa-gun, Iwate 029-4503, Japan
TEL +81-197-44-5121

Japan
PMDA
Oct. 2018
USA
FDA
Mar. 2019
EU
EMA
Jan. 2012
South Korea
MFDS
Jul. 2009
Saudi Arabia
SFDA
Jan. 2010
Taiwan
TFDA
Aug. 2013
Brazil
ANVISA
May. 2009
South Africa
MCC
May. 2013
Cephem Antibiotics API/Intermediates Manufacturing Building
We manufacture high quality Cephem antibiotics API and intermediates. In Compliance with J-GMP, c-GMP, EU-GMP, and PIC/S GMP, the plant is capable of supplying products globally to the USA, EU and Asian countries in addition to domestic market.

Production Control System
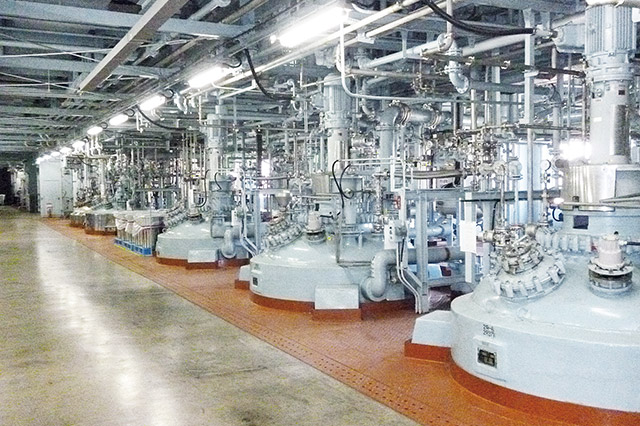
Multiple Large Capacity Reactors
General Work Area Major Facilities
| Reactors | 300~10,000L(SUS,GL) |
|---|---|
| Low Temperature Reactors | 10,000L(GL,-80˚C) |
| Separators | Centrifuge (Low Exhaust type): 60x40inch,48×38inch(TL) Centrifuge (Top Exhaust type) 30inch (TL) Pressurized Filtration Equipment: 1-6 m2(SUS) |
| Rectifying Column | φ1,200×6,000(TL) Continuous Rectifying Column: φ850×5,000(SUS) |
| Adsorbing Column | φ850×2,500(TL) |
| Dryer | Conical: 5,000L(GL), 6,000L(GL) |
General Work Area Major Facilities
| Reactor Crystallizer | Reactor: 500L (GL) Crystallizer: 1,000L (GL) |
|---|---|
| Separator | Centrifuge (Top Exhaust type): 42inch (TL) Centrifuge (Horizontal type): φ800(SUS) Pressurized Filtration Equipment: 0.5m2(TL) |
| Dryer | Conical: 300L(GL) 6,000L(SUS) Fluidized Bed Granulator: Container Capacity 790L (SUS) Filtration Dryer: 4m3(SUS) |
| Pulverizer | Power Mill Oscillator (SUS) |
Cephem Antibiotics Aseptic Formulation/Packaging Building
We have facilities for high quality cephem antibiotics injectable vial formulation (lyophilized formulation.) In compliance with J-GMP, c-GMP, EU-GMP, and PIC/S GMP requirements, we can manufacture high quality aseptic products for the global market.

Building

Three (3) units of largest-scale Lyophilizer in Japan
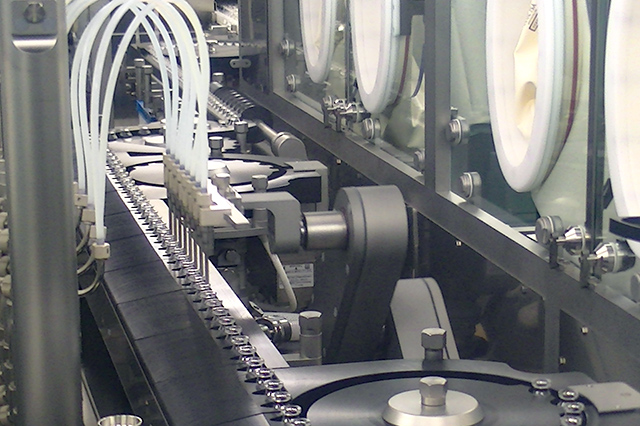
Vial filling line
Major Facilities
| Sterilization / Decontamination | Autoclave, VHP sterilizer |
|---|---|
| Solution preparation system | Solution preparation tank (350L, SUS), Automatic pH control system, Final mixing tank (1100L, SUS) Redundant sterilizing filtration system C/SIP systems |
| Vial Filling Line | Vial Washer, Depyrogenation tunnel, Vial filling stoppering machine (solution) RABS 300 Vials/min (φ24.5mm) |
| Lyophilizer | Lyophilizer, Automated vial loading systems 60,000 Vials/unit (φ24.5mm) |
| Vial Capper | RABS 300 Vials/Min (φ24.5mm) |
| Vial Exterior washing | Exterior Washer |
| Inspection | 100% visual inspection |
| Vial Packaging | Vial packaging line 200 Vials / min (φ24.5mm) (labeling, carton packaging, printing/inspection, weight check) Serialization system |
Cephem Antibiotics Solid Oral Formulation/Packaging Building
We have facilities for high quality cephem antibiotics solid oral formulation (tablets, capsules, granules.) In compliance with PIC/S GMP requirements, we can manufacture high quality products for the global market.

Tablet Press

PTP filling packaging line

PTP packaging line
Major facilities
| Mixing | Boat-shaped vibrating screen classifier Dome shaped mixer |
|---|---|
| Sifting/Classifier | Turbo cleaner Trituration sifting equipment |
| Granulator | Agitating granulator Fluidized-bed granulator dryer (30 type, 120 type) Cylindrical extrusion granulator Roller compactor Roll granulator Solution mixing tank (600L, 1,500L) |
| Dryer | Fluidized-bed dryer (120type) |
| Pulverization / Sizing | Jet Mill, Power Mill, Pin Mill FitzMill |
| Tableting | Tablet Press (45 stations) |
| Capsule Filling | Capsule filling equipment (200 type) Capsule weight inspection equipment Capsule exterior inspection equipment Capsule band-sealing equipment |
| Coating | Coating equipment (130 type) |
| Inspection | Exterior inspection equipment (granules) |
| Printer | Print inspection equipment (Tablet) |
| Printing Process | Poly bottle/Glass bottle filling packaging line (fine grains) Poly bottle filling packaging line (tablets) SP filling packaging line (fine grains/granules) Aluminum SP filling packaging line (Fine grains) PTP filling packaging line (capsules/tablets) Pre-stamper (variable GSI code) |
Carbapenem Antibiotics API/Intermediates Manufacturing Building
We have facilities for high quality carbapenem antibiotics formulation (API and intermediates.) In compliance with J-GMP, c-GMP, EU-GMP, and PIC/S GMP requirements, we can manufacture high quality aseptic products for the global market.

Aseptic isolator

Vibrating dryer
General Work Area Major Facilities
| Preparation Equipment | 1,000L(SUS) |
|---|---|
| Reactor | 300~4,000L(SUS,GL,HC) |
| Reactor Rectifier | 1,500~3,000L(SUS,GL) |
| Extractor | 1,000L(SUS) |
| Separators | Centrifuge: φ900(HC) Pressurized Filtration: 1.3m2(SUS) |
| Dryer | Conical: 1,000 (GL) |
Product Area major Facilities
| Preparation Equipment | 1,500L(SUS) |
|---|---|
| Reactor Crystallizer | Reactor: 500L(SUS) Crystallizer: 2,500L(SUS) |
| Solution Mixing Tank | 1,200L(SUS) |
| Aseptic Facilities | Depyrogenation ultrafiltration equipment: 50m2 Isolator:12.96m2 Autoclave: 1,300L(SUS) |
| Separators | Centrifuge: 30inch(TL) Pressurized filtration equipment: 0.15~0.78m2(SUS) |
| Dryer | Vibration Fluid Dryer: 120L(SUS) Fluid Dryer: 1,600L(SUS) |
Carbapenem Antibiotics Aseptic Formulation/Packaging Building
We have facilities for high quality carbapenem antibiotics injectables vial formulation (Aseptic powder filled formulation.) In compliance with J-GMP, c-GMP, EU-GMP, and PIC/S GMP requirements, we can manufacture high quality aseptic products for the global market.

Outside View

Vial Powder Filling facility
Major Facilities
| Sterilization/Decontamination | Autoclave, VHP sterilizer |
|---|---|
| Vial Filling Line | Vial Washer, Depyrogenation tunnel, Accofil model Powder filling stoppering machine, Capper, Exterior Washer RABS 120 Vials/min. (φ24.5mm, 29.5mm) |
| Inspection | 100% Visual Inspection |
| Packaging | Vial packaging line 110 Vials/Min. (φ24.5mm, 29.5mm) (labeling, carton packaging, printing/inspection) |
Cancer Pain Treatment Drugs API/Formulation (solid/injectables) / Packaging Building
We have manufacturing facilities for high quality cancer pain treatment drugs. In compliance with domestic API GMP, we can manufacture high quality products including injectable ampule formulation and solid oral formulation (tablets, powders.)




