EHS Management
■ EHS Organizational Structure
In the Shionogi Pharma EHS organizational structure, which promotes EHS (Environment, Health, and Safety) activities, the Corporate Officer in Charge of EHS performs integrated management of EHS for the entire company, and the plant manager of each site serves as the EHS Representative for the site.
In addition, the EHS Committee has been established as a deliberative body related to EHS. Comprising the heads of all organizations as well as EHS Management Representatives, who are responsible for practical EHS operations at each site, it formulates company-wide action targets related to EHS and promotes management reviews and other activities. Matters that have been discussed are subject to approval by the appropriate decision-making meeting body.
Moreover, a toxicologist is assigned as a person in charge of toxicity to evaluate the toxicity of chemical substances for scientific and rational measures against worker exposure.
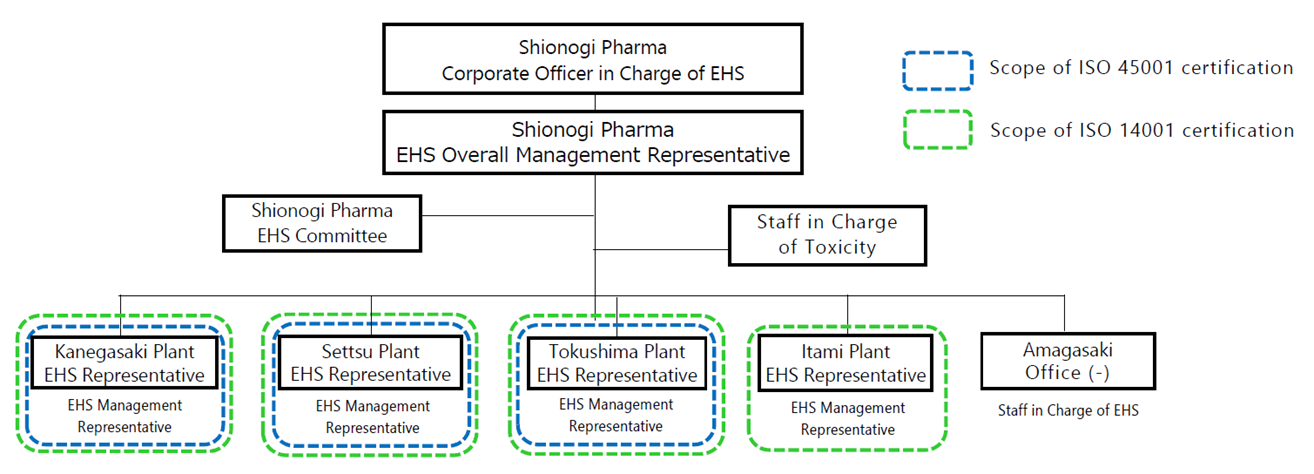
■ EHS Management System
Shionogi Pharma’s manufacturing plants are ISO (International Organization for Standardization) certified. As for the ISO 14001 environmental management system, we conduct organizational/corporate activities that are internationally recognized as being friendly to the global environment. Meanwhile, for the ISO 45001 occupational safety and health management system, we have established a mechanism for realizing a safe working environment in all workplaces. The certification status of each site is as shown in the figure above.
We check and review whether our activities are carried out in compliance with these frameworks through self-inspection conducted annually at each site.
■ Supply Chain Management
● Basic Concept of Procurement
As a member of the SHIONOGI Group, we have formulated the SHIONOGI Group Business Partner Code of Conduct*1 based on the principles of PSCI,*2 an NPO that aims to promote sustainable procurement in the pharmaceutical industry, and on the Ten Principles in four areas (human rights, labor, the environment, and anti-corruption) of the United Nations Global Compact,*3 and promote CSR procurement based on sincerity, accuracy, fairness, and transparency.
We ask our business partners to agree to comply with the SHIONOGI Group Business Partner Code of Conduct and to cooperate with us to autonomously and continuously improve our ESG management.

● ESG Management in CSR Procurement
Based on the SHIONOGI Group Procurement Policy, we work together with our business partners to ensure a stable and secure supply of high-quality pharmaceuticals while complying with laws and regulations, selecting suppliers in a rational manner, conducting fair and impartial transactions, and developing together with them.
In order to create products and services with excellent quality, safety, and medical economy that are useful to many patients, medical professionals, and society, and to provide them in a continuous and stable manner, we procure an appropriate amount and quality of raw materials, goods, and services at appropriate prices.In order to create products and services with excellent quality, safety, and medical economy that are useful to many patients, medical professionals, and society, and to provide them in a continuous and stable manner, we procure an appropriate amount and quality of raw materials, goods, and services at appropriate prices.
To this end:
• We add EHS items to our Supplier and Contractor Management Standards to strengthen ESG management of the entire supply chain, including business partners.
• We continue to work to ensure thorough compliance with laws, regulations, and other standards.
• We agree with the purpose of the “White Logistics” promotion campaign, in which the Ministry of Land, Infrastructure, Transport and Tourism, the Ministry of Economy, Trade and Industry, and the Ministry of Agriculture, Forestry and Fisheries are calling on listed companies and major companies in each prefecture to participate, and work on initiatives for “White Logistics.”
• As part of our efforts to reduce CO2 emissions, we select the top 20 companies by transaction value with the SHIONOGI Group as priority suppliers and work together with them, aiming to reduce CO2 emissions by 20% (compared to FY2019) by 2030.
