Climate Change and Environmental Protection Activities
■ CO2 Emissions Trends
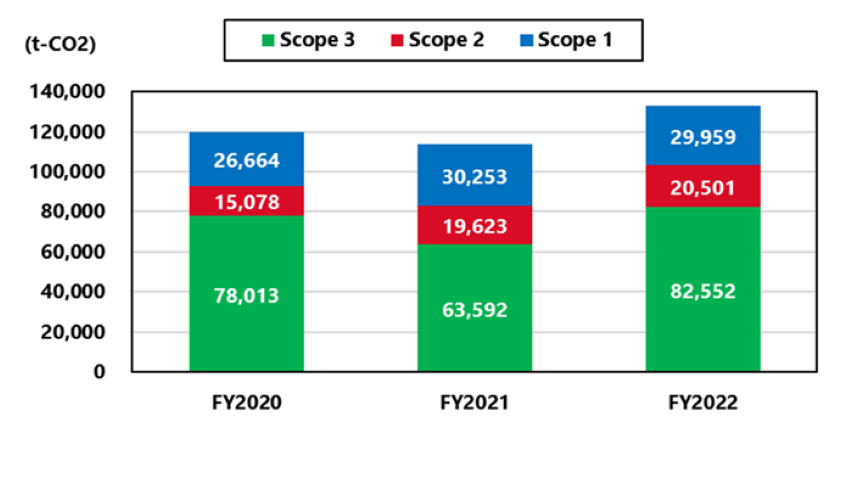
Trends of CO2 emissions in Scope 1 and 2
We set long-term targets for CO2 emissions and strive to continuously reduce the emissions. The trends of CO2 emissions in Scope 1 and 2 over the past three years are shown below. Due to the absorption-type merger of Nagase Medicals Co., Ltd., CO2 emissions from the Itami Plant have been added since FY2021. Even after deducting this effect, total CO2 emissions increased 9% from FY2020 to FY2021, and increased 2% from FY2021 to FY2022. We think that these increases were due to the launch of operation of the new high-potency manufacturing facility and an increase in contract manufacturing orders. On the other hand, as part of our efforts to reduce CO2 emissions, we are promoting the introduction of highly efficient equipment. The reduction effect of this initiative in FY2022 was approximately 280 t-CO2.
CO2 emissions in Scope 3
CO2 emissions in Scope 3 are higher than those in Scope 1 and 2. Future efforts are necessary to reduce these. (See “Supply Chain Management.”)
Scope 1: Direct greenhouse gas emissions by the reporting company itself (fuel combustion, industrial processes)
Scope 2: Indirect emissions associated with the use of electricity, heat, and steam supplied by other companies
Scope 3: Indirect emissions other than those in Scope 1 and 2 (emissions by other companies related to the company’s activities)
Trends of company-wide CO2 emissions (unit: tons-CO2)
| Trends of CO2 emissions in Scope 1 and 2 at each site (unit: tons-CO2) | |||
|---|---|---|---|
Site name / FY |
2020 |
2021 |
2022 |
Settsu Plant |
9,191 |
10,270 |
9,787 |
Kanegasaki Plant |
29,286 |
29,823 |
31,598 |
Tokushima Plant |
3,266 |
5,393 |
5,025 |
Itami Plant |
(3,861) |
4,389 |
4,051 |
● Future initiatives
In addition to working to reduce energy consumption by introducing highly efficient equipment, we will consider switching to electricity derived from renewable energy.
■ Environmental Protection Activities (preservation of biodiversity, protection and restoration of natural species)
As one of our efforts to contribute to environmental protection activities and the preservation of biodiversity, we are expanding with Suntory Holdings Limited to create original vending machines into all of our sites. By incorporating the activity to conserve and manage threatened species and medicinal plants,*1 conducted at the SHIONOGI Group’s Aburahi Botanical Gardens, and the Reforesting Kombu Project,*2 conducted by Shionogi Healthcare Co., Ltd., into the designs of vending machines, we aim to visualize these projects and raise employee awareness by making them think about environmental conservation at random moments in their daily lives. We also donate part of the sales from the vending machines to related external environmental protection groups to contribute to resolving social issues.
*1 At the Aburahi Botanical Gardens, we are involved in conserving over 1,000 threatened species and rare plants. We are also attempting to breed species of plants that are in danger of extinction ex-situ or at the Gardens and then return them to their own habitat.
*2 Shionogi Healthcare has begun switching its product raw materials from natural to farmed Gagome kombu since 2019, aiming to reduce the use of natural Gagome kombu to zero by 2024. They work to conserve and restore natural Gagome kombu by improving the breed of farmed Gagome kombu and creating a system for growing and circulating.

☆ Original design for Aburahi Botanical Gardens ☆
“Conservation and management of threatened species and medicinal plants”

☆ Original design for Shionogi Healthcare ☆
“Reforesting Kombu Project”
TOPICS
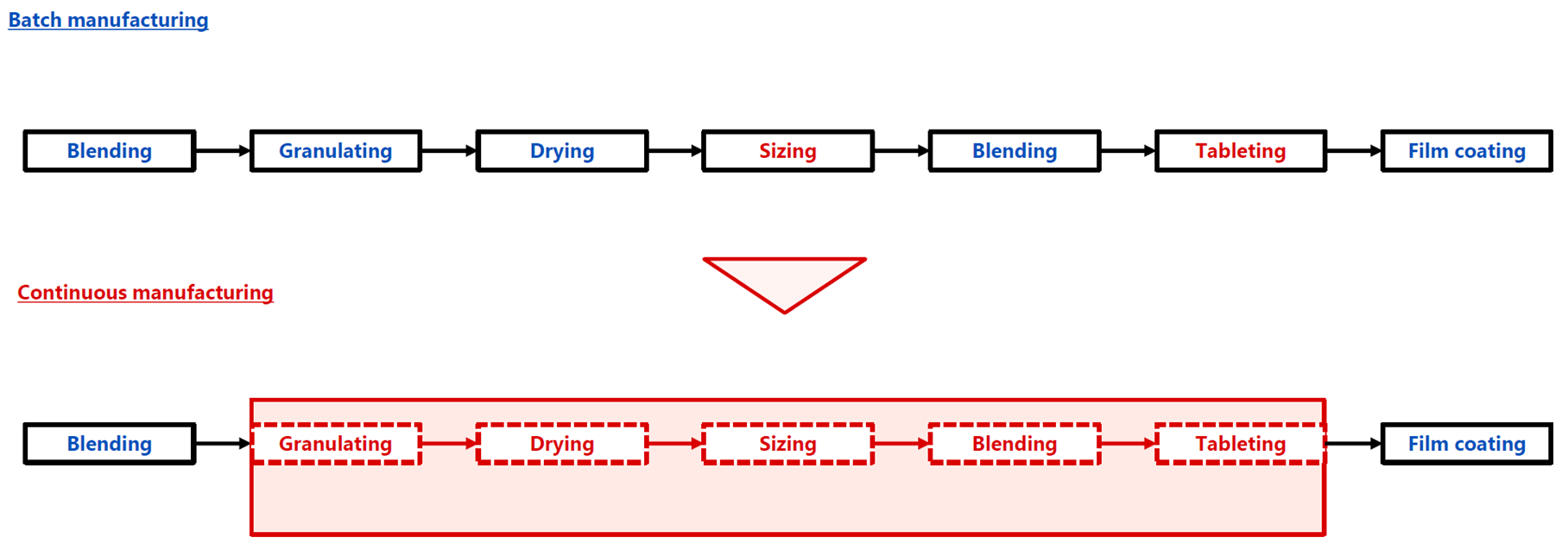
■ Continuous Manufacturing
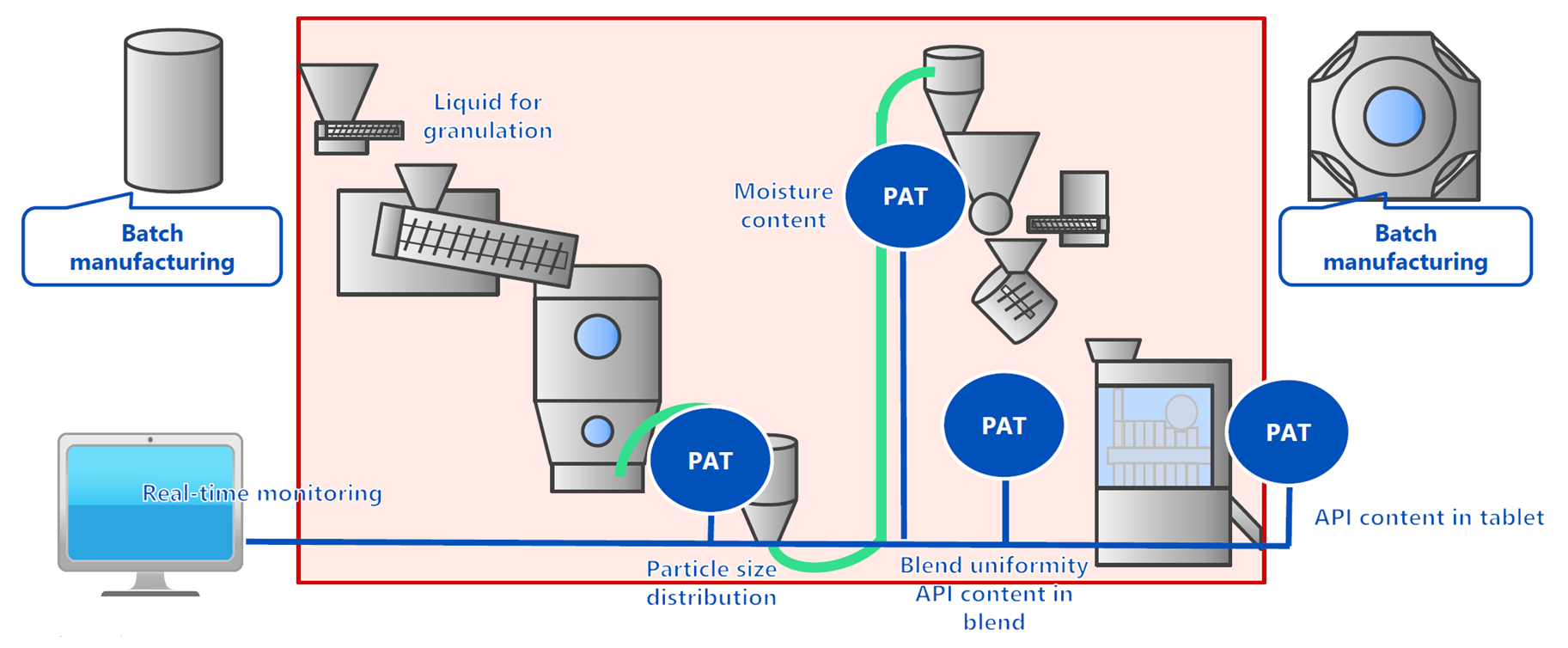
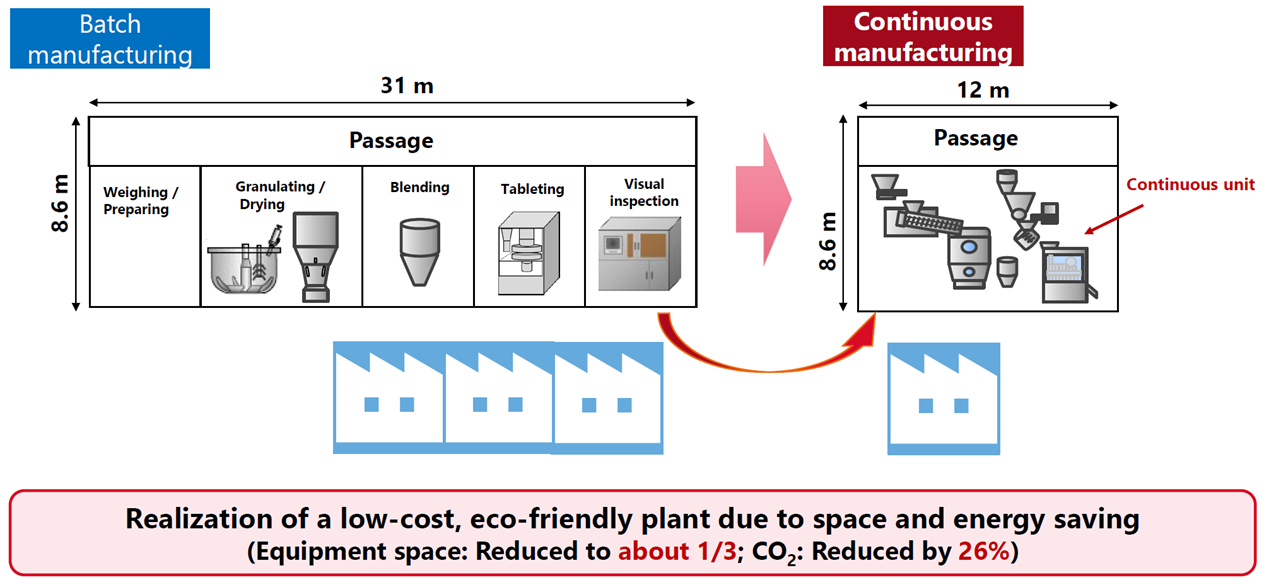
Continuous manufacturing of pharmaceuticals is a method that continuously manufactures products by feeding raw materials or their mixtures into the manufacturing process continuously while it is in operation. In general, the reasons why continuous manufacturing of pharmaceuticals (formulations) is required are as follows: (1) Since a scale-up study is unnecessary at the development stage, it is possible to reduce the amount of APIs and shorten the manufacturing process development period; (2) The use of Process Analytical Technology (PAT) and other tools makes it possible to monitor the manufacturing status, allowing for more reliable manufacturing; (3) The lot size can be changed flexibly, which contributes to securing a stable supply of pharmaceuticals and reducing the amount of product waste; and (4) Since manufacturing is possible with smaller equipment compared to batch manufacturing, it is possible to save space and energy and reduce CO2 emissions.
The following diagram is an example of continuous manufacturing in which five processes from granulating to tableting are performed continuously in the tablet manufacturing process. Shionogi Pharma has actually applied for approval for continuous manufacturing with these five processes, has obtained approval, and has succeeded in their practical application.
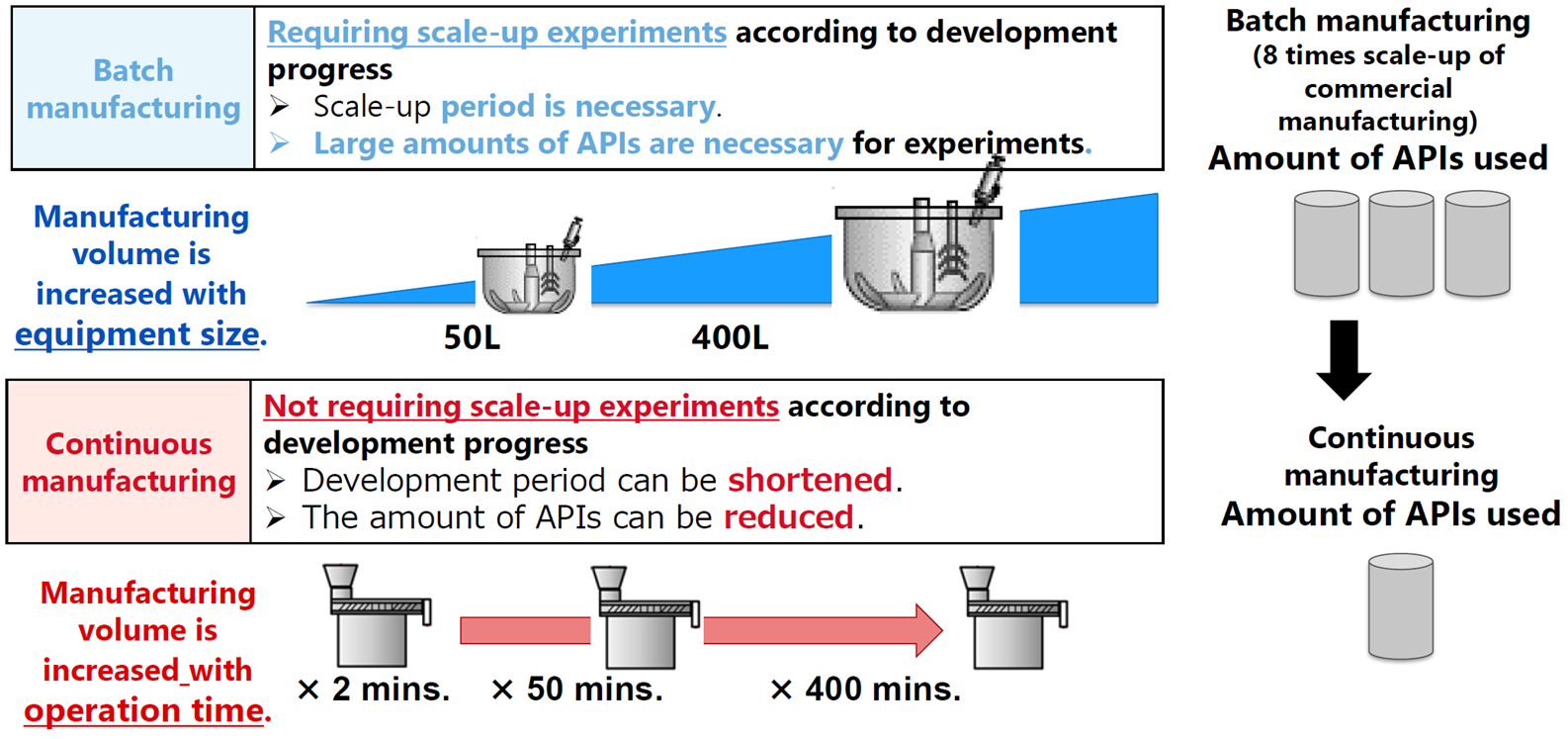
● Reduction of the amount of APIs used
In our example, the adoption of continuous manufacturing enabled us to significantly reduce the amount of APIs necessary for a scale-up study (see the diagram below). This leads to a reduction in CO2 emissions, equivalent to the amount of APIs that could be reduced, in the API manufacturing process.
Comparison between batch manufacturing and continuous manufacturing
(amount of APIs used)
● Reduction of CO2 emissions
In addition to reducing the amount of APIs, continuous manufacturing can also reduce the energy used in the manufacturing process. The results of our internal investigation show that CO2 emissions have decreased by 26% in continuous manufacturing compared to batch manufacturing. In addition, it can be said that continuous manufacturing has less environmental impact since construction materials can be reduced due to space saving.
Comparison between batch manufacturing and continuous manufacturing
(space saving)
● Continuous Manufacturing Facilities
For continuous manufacturing, we have facilities for investigational drug manufacturing (the left image below) and commercial production facilities (the right image below).
● Future Plans
We aim to obtain further approval for our products and acquire new contract manufacturing orders through the application of continuous manufacturing.

